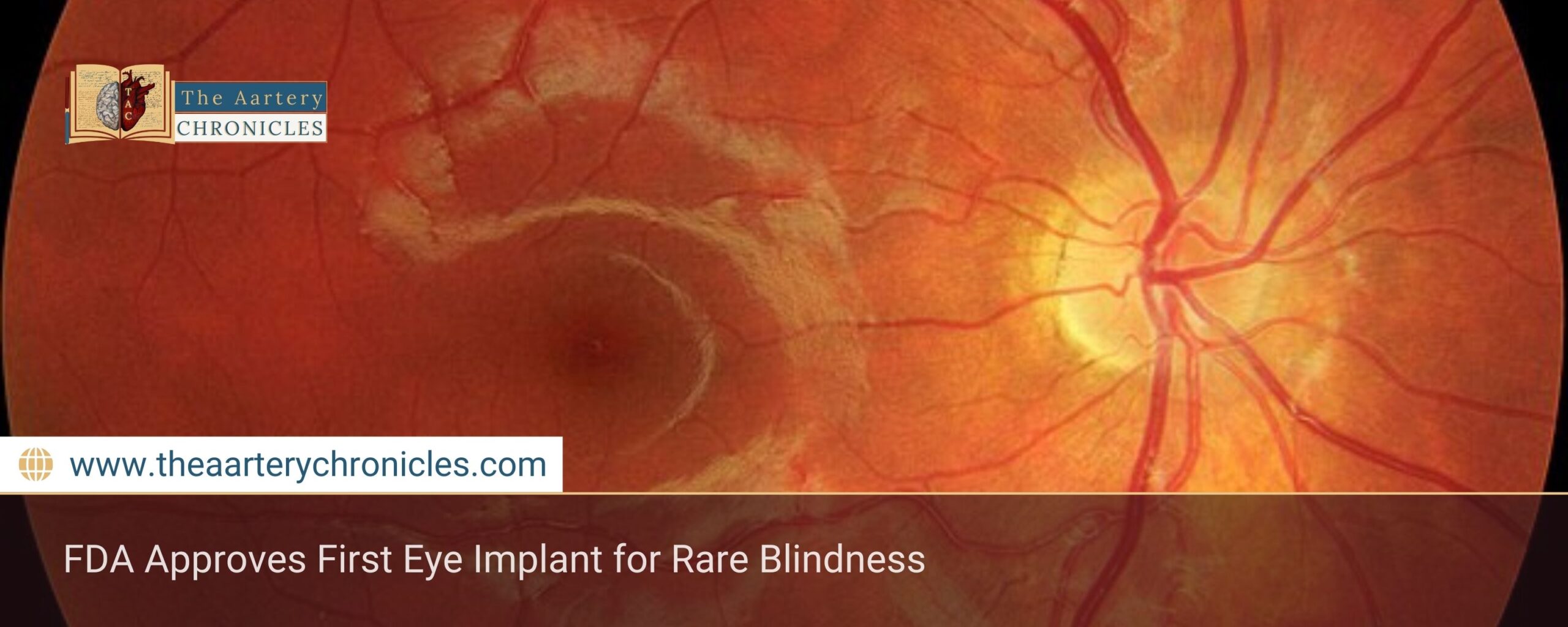
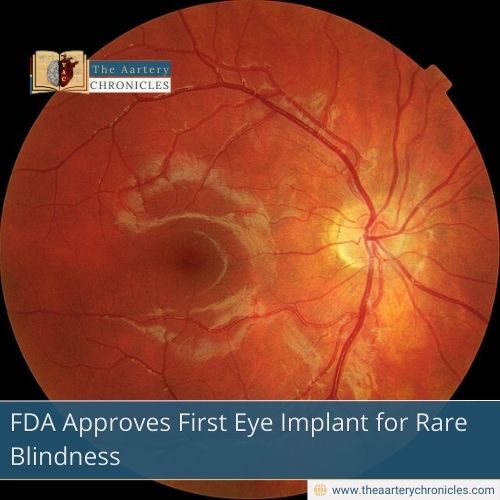
FDA Approves First Eye Implant for Rare Blindness
Summary: ENCLENTO, a tiny eye implant, just became the first FDA-approved treatment for macular telangiectasia type 2 (MacTel), which is a pretty rare retinal condition. It was tested through Phase 3 trials and is a pretty exciting breakthrough—this cell-based implant helps slow down vision loss by saving those critical retinal cells.
This Could Be a Big Break for People with a Rare Eye Condition
If you have macular telangiectasia type 2 (MacTel), a rare eye disease that slowly steals your central vision, you probably haven’t had many options for treatment. But now, there’s some real hope.
ENCELTO, a tiny implant placed in the eye, is actually the first FDA-approved treatment for MacTel. This rare condition, often called an orphan disease and considered a neurodegenerative retinal disorder, affects thousands of people around the world and can lead to permanent blindness.
What exactly is MacTel, and why does it make things so tricky?
MacTel, short for macular telangiectasia type 2, is a pretty rare eye condition. It’s considered an orphan retinal disorder, meaning it’s not very common and doesn’t get much attention. It messes with your photoreceptors, the light-sensing cells in your eye that help you see clearly, especially when it comes to central vision. Over time, these cells start to break down, making it harder to read, drive, or even recognize faces. Up until now, there hasn’t been any approved treatment to stop or slow down this process.
Understanding ENCELTO: Its Mechanism
Created by Neurotech Pharmaceuticals and evaluated in partnership with Scripps Research, ENCELTO is a cell-based neuroprotective implant positioned at the back of the eye. It works by providing a continuous release of ciliary neurotrophic factor (CNTF)—a critical protein that supports retinal neuron health and guards against degenerative processes.
The device comprises retinal pigment epithelial cells that have been genetically modified enclosed within a small collagen capsule. This design prevents immune system rejection while ensuring long-lasting delivery of CNTF. Consequently, it offers ongoing protection to delicate retinal cells, decreasing the likelihood of vision loss and blindness.
Inside the Study: Who and How
Two phase 3 clinical trials brought in a total of 228 patients from 47 different spots around the world. Over the course of 2 years, ENCELTO really made a difference by slowing down the loss of photoreceptors when compared to eyes that got the fake treatment:
- 54.8% less decline in one trial
- 30.6% less decline in the other
“This is a step toward redefining how we think about vision loss,” says Professor Martin Friedlander of Scripps Research. “Instead of waiting for cells to die, we’re learning how to protect and preserve them.”
Functional Vision Outcomes: Mixed but Promising
Although ENCELTO demonstrated notable success in maintaining the structural integrity of the retina, the observed functional outcomes such as reading speed and retinal sensitivity, presented mixed results. Nonetheless, microperimetry assessments revealed a statistically major deceleration in the progression of vision loss in one of the trials. When the data from both trials were combined for analysis, the overall benefit became evident.
Safety and Tolerance
The implant was well-tolerated with minimal side effects. Interestingly, its benefits appeared regardless of baseline vision or disease stage, but early intervention may yield the best results.
FDA Approval and Future Applications
The success of these trials led to FDA approval in March 2025, making ENCELTO:
- The first therapy for MacTel
- The first cell-based neuroprotective treatment for any retinal disease
Researchers are now exploring its potential for other neurodegenerative eye diseases. This platform could revolutionize treatments for conditions where protecting nerve cells is crucial.
What This Means for Patients
ENCELTO brings new hope to those living with MacTel, proving that neuroprotection can slow vision loss. Future studies aim to refine treatment timing and identify patient subgroups for maximum benefit.
Takeaway
The FDA approval of ENCELTO marks a historic moment in ophthalmology. For patients with MacTel, this tiny implant offers a chance to preserve vision, turning the tide against a once-untreatable disease. As research continues, this breakthrough could pave the way for therapies for other blinding conditions.
Dane
I am an MBBS graduate and a dedicated medical writer with a strong passion for deep research and psychology. I enjoy breaking down complex medical topics into engaging, easy-to-understand content, aiming to educate and inspire readers by exploring the fascinating connection between health, science, and the human mind.