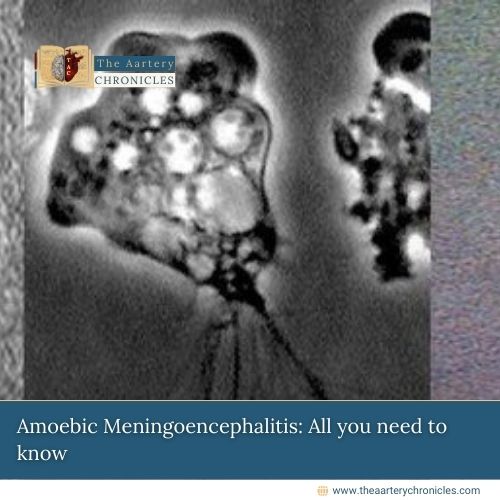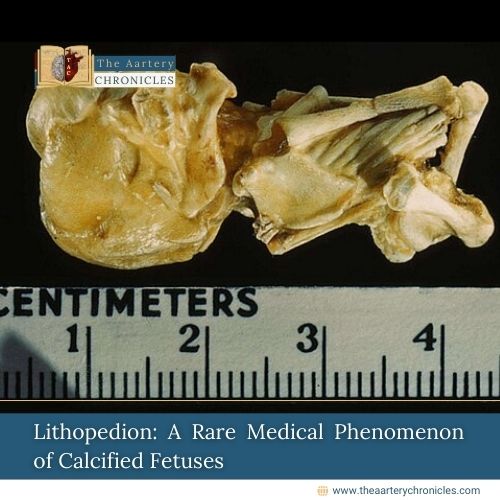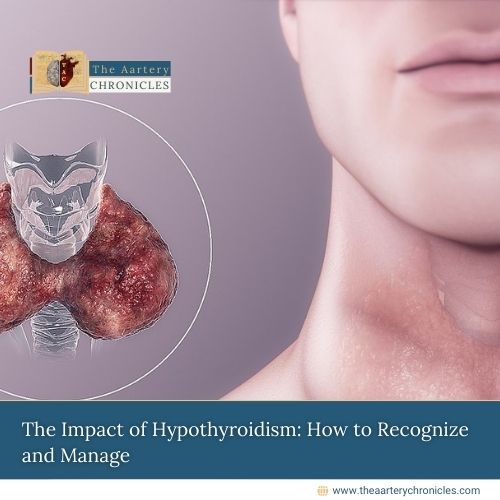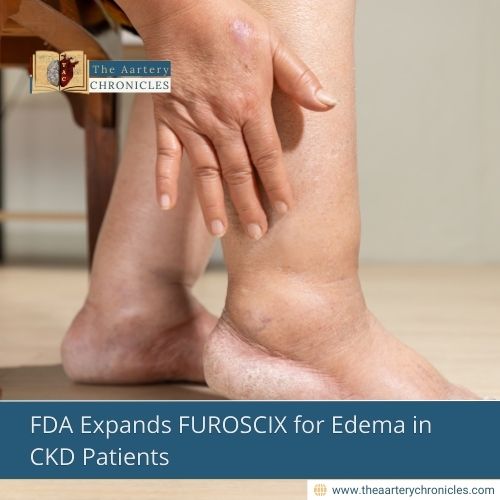
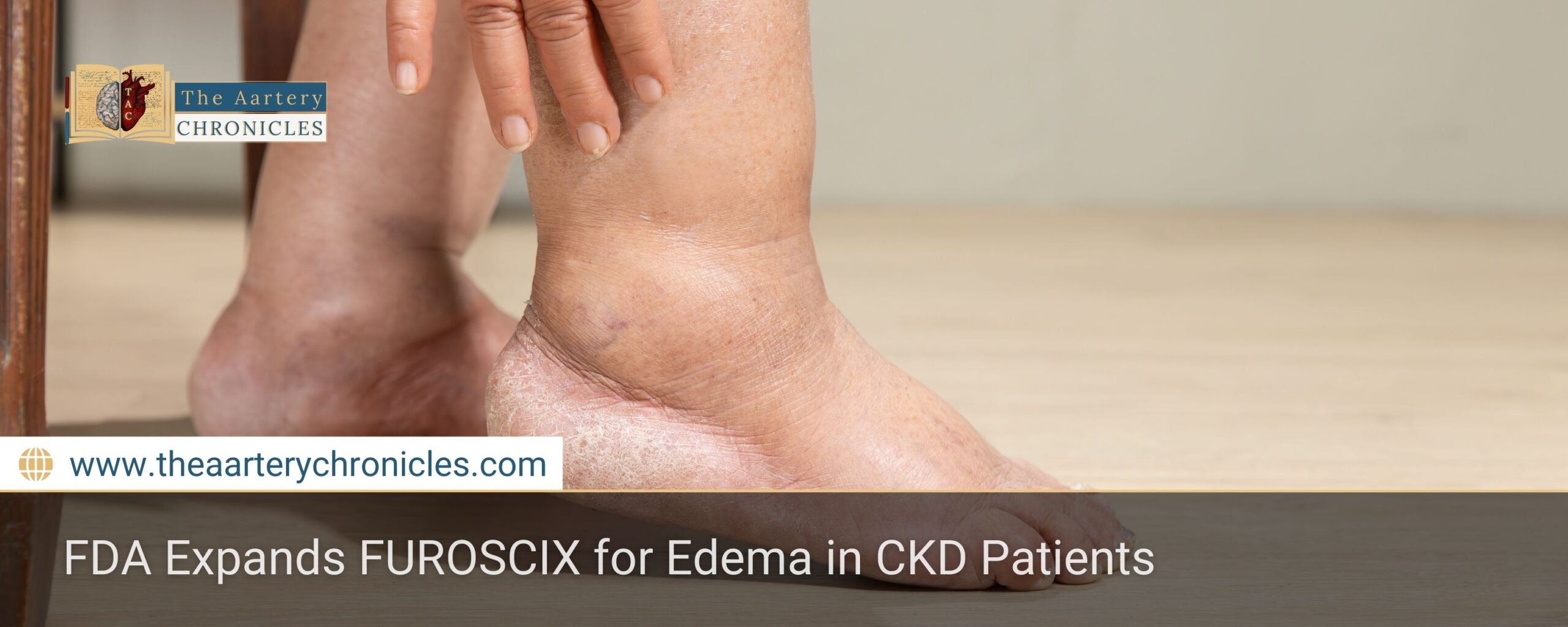
FDA Expands FUROSCIX Approval for Edema in CKD Patients
Summary: The FDA has expanded FUROSCIX (furosemide injection) approval to include edema treatment in chronic kidney disease (CKD) patients, including those with nephrotic syndrome. This move enhances treatment options for fluid overload, potentially reducing hospital visits.
A Breakthrough for CKD Patients: FDA Expands FUROSCIX Use
Managing fluid overload in chronic kidney disease (CKD) just got easier! The U.S. Food and Drug Administration (FDA) has officially expanded the approval of FUROSCIX (furosemide injection) to treat edema in CKD patients, including those with nephrotic syndrome. This means better care options and potentially fewer hospitalizations for patients struggling with fluid retention.
“This milestone marks a significant step forward for the FUROSCIX franchise and underscores our commitment to addressing unmet needs of cardiorenal patients,” said John Tucker, CEO of scPharmaceuticals.
With this expansion, nephrologists now have an additional tool to manage fluid overload in CKD and heart failure patients, helping them maintain euvolemia (balanced fluid levels) without frequent hospital visits.
Why FUROSCIX is important for CKD & Heart Failure Patients
FUROSCIX is a subcutaneous furosemide injection (80 mg/10 mL) designed to treat fluid overload (edema) in adults with:
- Chronic kidney disease (CKD), including nephrotic syndrome
- Chronic heart failure (CHF)
This expansion is a game changer because FUROSCIX allows patients to manage fluid overload at home, reducing the burden on healthcare facilities.
“Expanding the FUROSCIX indication to CKD patients gives clinicians an essential tool to manage fluid overload more effectively,” said Dr. Suneel Udani, nephrologist at NANT.
FUROSCIX: How It Works & Key Benefits
FUROSCIX delivers furosemide subcutaneously, offering a more convenient alternative to IV diuretics. The benefits include:
- Reduced hospital admissions by enabling at-home treatment
- Improved symptom management for edema-related congestion
- Faster action to restore fluid balance and prevent complications
FUROSCIX: Important Safety Information
FUROSCIX is a promising treatment, but it comes with some important safety considerations:
Contraindications:
- Not for patients with anuria (lack of urine production)
- Avoid in those with hypersensitivity to furosemide or its components
Potential Side Effects:
- Electrolyte imbalances (monitor sodium, potassium, and kidney function)
- Dehydration & low blood pressure (especially in elderly patients)
- Ototoxicity (hearing issues) in high doses or rapid injections
- Urinary retention in patients with prostate or bladder issues
Patients should also be aware that contact with water or excessive movement during use may affect the On-body Infusor’s performance.
Final Thoughts
The FDA’s expanded approval of FUROSCIX for CKD patients marks a significant advancement in fluid overload management. With its ability to provide effective diuresis outside of hospital settings, it offers a new level of convenience and relief for patients with CKD and heart failure.
If you or a loved one is struggling with fluid retention due to CKD or CHF, consult your doctor to see if FUROSCIX could be the right treatment option.
Inputs from various media sources
Dane
I am an MBBS graduate and a dedicated medical writer with a strong passion for deep research and psychology. I enjoy breaking down complex medical topics into engaging, easy-to-understand content, aiming to educate and inspire readers by exploring the fascinating connection between health, science, and the human mind.